
General FAQs
Why are honey, arabic gum, cellulose, and calcium stearate added to products?
Honey and arabic gum are used in our tablet manufacturing process. Cellulose is often used to give body to the powders. Calcium stearate, derived from vegetable sources, is used as a lubricant to help products release from the tooling of the tablet and capsuling machines. No artificial flavoring is ever used.
Why would a product look different from bottle to bottle?
Because of the variability in sources of natural and organic ingredients, color, texture, and odor may vary from batch to batch. However, the basic formula remains constant, and quality is ensured by repeat testing.
Is your calcium stearate derived from hydrogenated oils? Is it safe for consumption?
The calcium stearate we use is derived from palm oil, which is a saturated fat, like cocoa butter, dairy fats, etc. It is approved by the FDA to be used as a lubricant and is approved for use in food products.
Is the gelatin used to make your capsule and soſtgel products derived from animal material?
Yes. The gelatin is derived from bovine and/or porcine sources. Cellulose is used to make vegetarian capsules.
Are the gelatin capsules considered pareve/parve?
Yes. All of our gelatin capsules are certified kosher or pareve/parve. Pareve/parve is a classification under Jewish dietary law meaning the product has been processed so extensively that it can be considered to have been prepared without meat, milk, or other derivatives.
If the client is concerned about gelatin consumption, is there any way for that client to still receive the benefits of these products?
Yes. Some clients open the capsules and mix the contents into a food or beverage source, such as applesauce, yogurt, or a nutritional shake.
Which Standard Process supplements should not be chewed and why?
Zypan, Betaine Hydrochloride, and Cal-Amo should never be chewed because the acids may cause potential damage to tooth enamel. Chlorophyll Complex softgels will temporarily stain the teeth and turn the tongue green.
Why is it recommended that some Standard Process supplements be chewed?
Some Standard Process supplements are designed as chewables for those who cannot swallow capsules or tablets. After chewing, taste-bud receptors tell the brain what is entering the digestive system. Tasting is considered the first stage of digestion, when salivary secretion enzymes are activated.
What allergens might be found in Standard Process products?
Our products are processed in a facility that manufactures other products containing soy, milk, eggs, wheat, peanuts, tree nuts, fish, and shellfish. The preceding statement is printed on every product label to alert extremely sensitive clients. Please read our labels carefully for any possible ingredient allergens before recommending supplements to clients who are sensitive to certain foods. While we take precautions to avoid cross-contamination by thoroughly cleaning and sanitizing equipment and cleaning production suites, it is possible that some allergen residue may be present.
Does the nutritional yeast in Standard Process products cause yeast infections?
Nutritional yeast, S. cerevisiae, is not pathogenic and does not cause candida or other yeast infections.
How do I read “Best By” and “Lot Code” information on Product Boxes and Bottles?
For 90 years, Standard Process has been committed to making high quality whole food-based supplements that change lives. Please refer to the “Best By” date on all product boxes and bottles to determine how long a product maintains optimal quality and/or flavor. Standard Process only guarantees product potency through the “Best By” date.
Additionally, Standard Process no longer advises healthcare practitioners and clients to use product lot codes to verify when a product was produced. In early 2019, the company implemented a new Enterprise Resource Planning system that randomly generates lot codes for internal production traceability only. Due to varying degrees of potency, quality and/or flavor of product ingredients overtime, the “Best By” date continues to serve as the best reference for customers.
How do you read your product labels?
Below is an example of the different parts of our label and what they refer to.
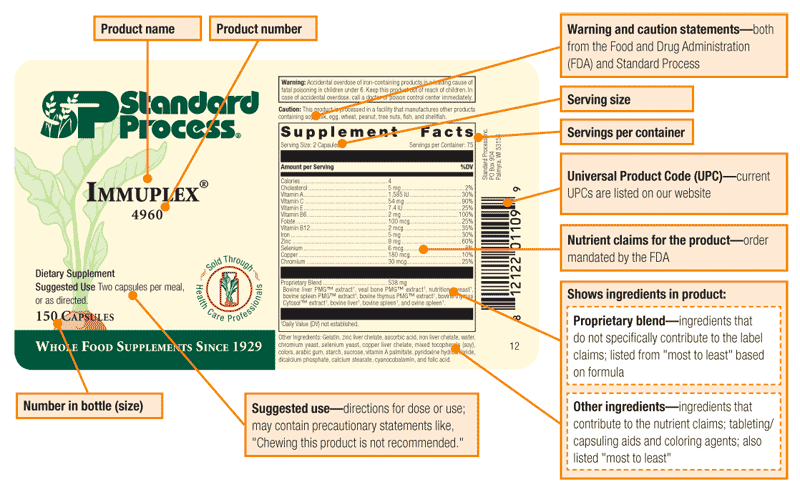
Are any of your products made with peanut ingredients?
Only Peanut Butter Standard Bars continue to be made with peanut butter. Recognizing the severity of peanut allergies, Standard Process has removed peanut bran from its products and replaced it with rice bran.
Why do you have sucrose on your product labels?
The sucrose found in our products comes from natural sources such as beets and sugar cane. Sucrose, along with starch and arabic gum, is used to coat vitamin A and vitamin D (cholecalciferol), making them into a powdered form that helps prevent oxidation of these vitamins. The amount of sucrose in the actual product is very minimal.
Which products contain shellfish?
Nutrimere and Iodomere contain green-lipped mussel. Glucosamine Synergy contains glucosamine sulfate that is derived from crab and shrimp. While not a shellfish, calamari (the source of oil in Calamari Omega-3 Liquid) is in the same family and may trigger a reaction in those who are allergic to shellfish. Please consult the product labels for the complete ingredient listing on each product.
Which of your products contain soy? Should I worry about an allergic reaction?
Some products contain soybean ingredients, such as soy powder, soy nuggets, and soy protein. Soybean lecithin, a source of choline and B vitamins, is found in other Standard Process products. It acts as an emulsifier, keeping water and fats from separating in foods. Individual sensitivities to soy protein should be discussed with your clients.
Why is it better to take supplements throughout the day instead of all at once?
The body processes most compounds based on concentration; so the more there is, the faster your body works to process it. The key is getting the right concentration at the right time. Taking smaller amounts throughout the day allows the body to maintain a lower but more consistent amount for a longer period of time. It is more efficient for your body and more effective in sustaining the molecular mechanisms supported by the compounds in our supplements.
Do you test for contaminants in your marine oils?
Consistent with our commitment to produce and sell only the highest-quality dietary supplements, each batch of marine oil is tested for potential oxidation, mercury, polychlorinated biphenyls (PCBs), and dioxin. The product is also tested for bacteria and to verify our label claims. These tests are carried out by Standard Process and third-party labs. We use this process for testing Tuna Omega-3 Oil, Tuna Omega-3 Chewable, and Calamari Omega-3 Liquid.
During manufacturing, our marine oils are processed to retain their natural triglyceride structure.
We also take great care in sourcing our marine-oil products. Tuna comes from the abundant skipjack and/or yellowfin tuna populations. Our calamari oil is certified as sustainable by a nonprofit, nongovernmental organization, Friend of the Sea®.
Should Standard Process tablets dissolve in water or vinegar?
All products do meet the standard disintegration levels set forth by the United States Pharmacopeia (USP). Water and vinegar are not good models for how the stomach digests foods.
Does Standard Process have a list of gluten-free products?
Yes, visit here for products organized by dietary restrictions. Clients and health care professionals should know that we follow the FDA guidelines, which state that products that contain less than 20 parts per million (ppm) of gluten can be labeled as gluten-free. All of our products that are designated as gluten-free contain less than 20 ppm and are tested every time they are manufactured to be sure that they meet this standard.
Does Standard Process use Genetically Modified Organisms (GMOs)?
Standard Process is committed to the health-giving properties of whole food nutrition and using ingredients that have not been genetically modified. As part of this commitment, we first source ingredients from our own 623-acre certified organic farm. Certified organic farming does not allow for genetic engineering. A great majority of all raw plant ingredients used to make our products are grown on our farm. When sourcing ingredients we can’t grow on our farm, we are committed to selecting partners who offer ingredients that have not been genetically engineered. We rigorously evaluate all outside vendors and validate the raw ingredients they supply.
What do I need to consider when recommending a combination of products?
Standard Process products are thought to work synergistically and support multiple aspects of health. In some cases, combined products can result in very high levels of certain vitamins or minerals. Where warranted, guidance is provided for individual products on the label and in this catalog via warnings and cautions. Additional information about vitamin and mineral levels is provided through the “daily values” reported on the label. These values, set by the Food and Drug Administration, indicate how much of the recommended daily consumption of the nutrient is found in a serving. Several excellent resources detailing vitamin/mineral action and current knowledge regarding recommended levels are available online.
- Linus Pauling Institute
- Food and Nutrition Information Center
- Mayo Clinic
Why have some nutrient measurements changed from IU to mcg?
Label requirements recently changed for how some nutrient measurements are listed. Rather than measurements listed in IU many are now listed in mcg. See chart for sample conversions:
| Vitamin A/Beta-carotene | |
| To convert from IU of vitamin A or beta-carotene to mcg RAE (Retinol Activity Equivalents), multiply by 0.3 | |
| Previous Value | Updated Equivalent |
|---|---|
| 667 IU | 200 mcg |
| 1,667 IU | 500 mcg |
| 3,333 IU | 1,000 mcg |
| 6,667 IU | 2,000 mcg |
| Vitamin D3 | |
| To convert from IU of vitamin D3 to mcg of vitamin D3, multiply by 0.025 | |
| Previous Value | Updated Equivalent |
|---|---|
| 100 IU | 2.5 mcg |
| 200 IU | 5 mcg |
| 400 IU | 10 mcg |
| 800 IU | 20 mcg |
| 2,000 IU | 50 mcg |
| 4,000 IU | 100 mcg |
| Vitamin E | |
| To convert from IU of vitamin E to mg of d-alpha tocopherol, multiply by 0.67 | |
| Previous Value | Updated Equivalent |
|---|---|
| 75 IU | 50 mg |
| 149 IU | 100 mg |
| Folate | |
| To convert from mcg DFE (Dietary Folate Equivalents), divide by 0.6. This conversion applies to both folic acid and 5-MTHF | |
| Previous Value | Updated Equivalent |
|---|---|
| 400 IU | 667 mcg |
| 800 IU | 1,333 mg |
Manufacturing Questions
What type of binders do you use?
Honey, arabic gum, cellulose gum, and cellulose are used as binders. Calcium stearate, derived from vegetable sources, is used as a lubricant to help the products flow into the tablet machines.
Are calcium lactate and calcium stearate derived from dairy sources?
No, they are derived from vegetable sources.
What yeasts are used in your products?
The three types of yeast used in some of our products are:
Nutritional yeast
High chromium yeast
High selenium yeast
What are the capsules made of?
Most are made of gelatin, derived from animal sourced collagen. The others are made from vegetarian cellulose. The capsule itself is manufactured to result in a strictly kosher gelatin product.
Can the tablets be chewed?
Yes; however, some products include ingredients that may be harmful to teeth so it would be best to check with your health care professional.
Can the capsule products be taken in the powder form?
Yes; it is not necessary to leave the product in the capsule if you prefer to take the product in the powder form.
What is the shelf life of your products?
The shelf life on most of our products is between 1½ and 2 years. The “Best Used By” information, located on all product boxes and on the shoulder of all product bottles, represents the date by which the product is best used by and the product code. Standard Process only guarantees product potency through the “Best Used By” date. Products are continuously tested to determine their shelf life. This testing has shown that certain products tend to have shorter shelf lives, which is reflected in the “Best Used By” date.
Do products have a longer shelf life if they are unopened?
No, the shelf life is the same.
Who regulates the supplement industry?
Unlike what the pharmaceutical industry and some news media would have you believe, the supplement industry is indeed regulated and inspected by various governmental agencies. Standard Process Inc. is regulated and inspected by:
- The Food and Drug Administration (FDA)
- The United States Department of Agriculture (USDA)
- Wisconsin Department of Agriculture (WI-DA)
- Occupational Safety and Health Administration (OSHA)
- Midwest Organic Services Association (MOSA)
- Wisconsin Department of Natural Resources (DNR)
Why is there a caution statement now being placed on some product labels?
As a precaution, Standard Process will be adding the following warning statement to all product labels: This product is processed in a facility that manufactures other products containing soy, dairy, egg, wheat, peanut, tree nuts, fish, and shellfish. Standard Process has also added a similar warning on MediHerb products that are packaged in our facility. This warning is meant for people who may have severe allergies to these foods.
Standard Process follows good manufacturing practices to avoid cross-contamination–practices such as taking apart and cleaning all equipment between production runs. It’s just one way we can make our products safer for consumers.
Does Standard Process use Genetically Modified Organisms (GMOs)?
Standard Process is committed to the health-giving properties of whole food nutrition and using ingredients that have not been genetically modified. As part of this commitment, we first source ingredients from our own 623-acre certified organic farm. Certified organic farming does not allow for genetic engineering. More than 80 percent of all raw plant ingredients used to make our products are grown on our farm. When sourcing ingredients we can’t grow on our farm, we are committed to selecting partners who offer ingredients that have not been genetically engineered. We rigorously evaluate all outside vendors and validate the raw ingredients they supply.
How is the manufacturing equipment cleaned between different product runs?
Between each separate product run, any equipment that comes in contact with ingredients or finished product is disassembled and washed.
Cleaning agents are rotated monthly to avoid bacterial resistance.
Swab samples are taken from several areas of each piece of equipment.
These samples are exposed to a luminometer, which fluoresces in proportion to the amount of adenosine triphosphate (ATP) in the sample.
ATP is found in and around all biological matter and would indicate a possible bacterial presence.
If the luminometer measures ATP results beyond an acceptable level, the cleaning process is repeated and additional ATP tests are conducted.
This is done as many times as needed to obtain acceptable samples.
After the cleaned equipment passes the ATP testing protocol, the equipment is sanitized.
Only then is the equipment reassembled for use with a new batch of product.
If a clean piece of equipment is unused for more than 24 hours, it is resanitized prior to use.
In addition to the equipment itself, the surrounding production suite, including the floors, walls, and vent covers, is cleaned.
